The process of fusion of substances takes place during the conversion of a solid substance into a liquid. This occurs when the internal energy of the solid increases typically by the application of heat or pressure which increases the substances temperature to the melting point.
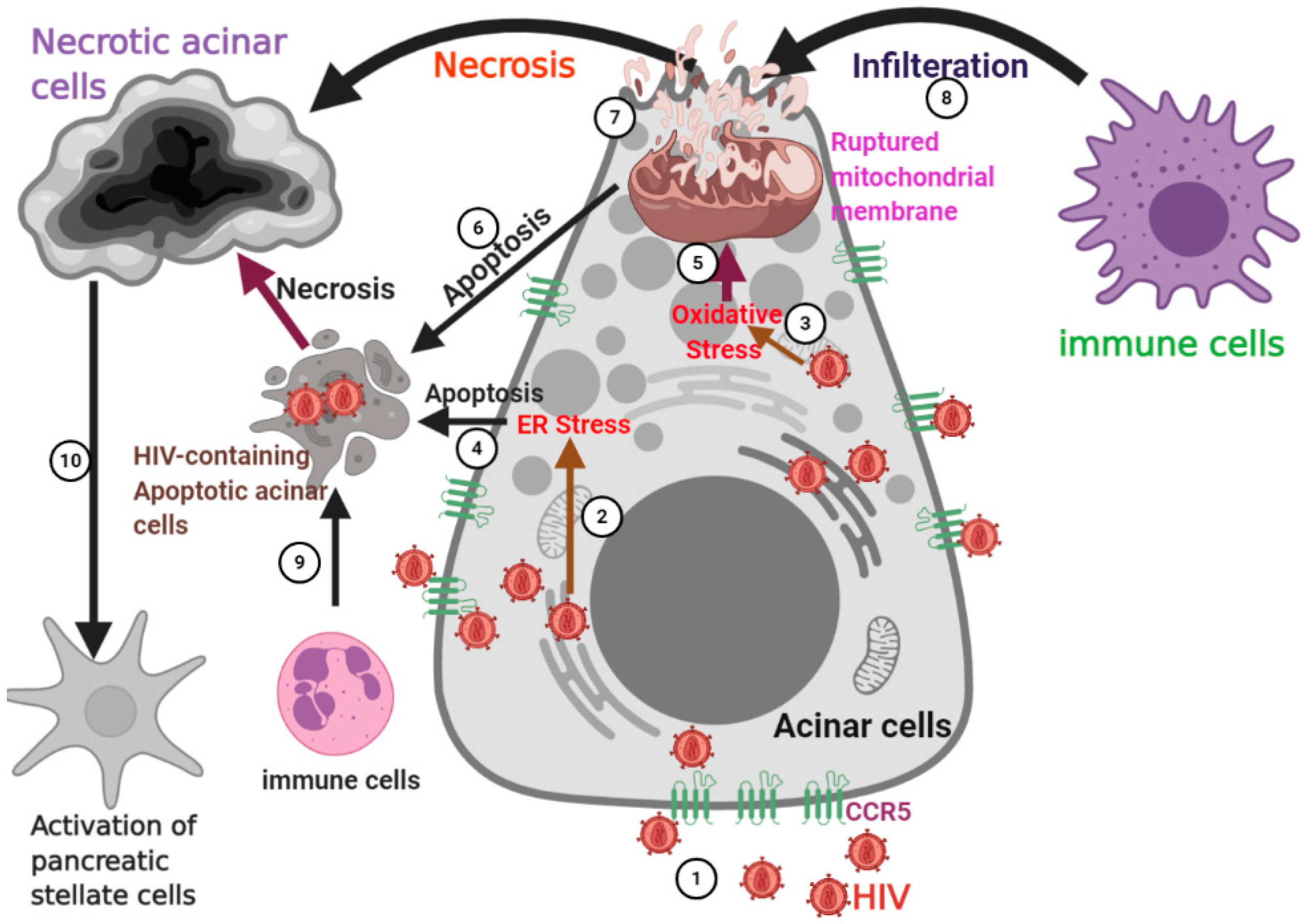
Biology Free Full Text Pancreatogenic Diabetes Triggering Effects Of Alcohol And Hiv Html
Must be added to 1 kg of a substance at its melting point.
. Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase. In this case when heat is applied to the system its temperature value will not change. When a substance undergoesfusionit a freezes.
When a substance boils it undergoes a process known as. What is the change in entropy of 1 kg of water when it is heated from 0C to 100C. This is important to every living thing on earthOct 22 2019.
When a pure substance undergoes a chemical change it is no longer that same substance. Find step-by-step Chemistry solutions and your answer to the following textbook question. The latent heat of fusion of a substance is LT and its melting temperature is Tm.
When a system undergoes phase changes such solid to liquid or liquid to gas the temperature of the system will remain constant. A chemical change changes the identity of the substance. Fusion- Direct change between a solid and liquid solid - liquidtransitions Melting solid - liquid or Freezing liquid - solid Most of the energy gained by a substance during a phase changegoes into the rearrangement of the molec.
Using the heat of vaporization of water 540 calg the specific heat of water and the heat of fusion of water 80 calg find the total heat in calories that are released when a 250 g sample of steam at 100 º Celsius condenses and cools to 0 º. What is fusion and sublimation. Latent heat of fusion is the heat that a.
Evaporation occurs at temperatures below the boiling point and occurs on the liquids surface. Will change the temperature of 1 kg of a substance with 1 Kc. Hope i helped Is heat the fusion that occurs in a.
Greater the heat of fusion of a substance higher the magnitude of intermolecular forces. Evaporation occurs at temperatures below the boiling point and occurs on the liquids surface. View the full answer.
When a substance undergoes fusion it melts. When a substance melts it undergoes a process known as 1 condensation 2 fusion 3 sublimation 4 vaporization. What does it mean when something is dissolved.
Complete the following statement. What is the process of melting also called. What is the process of melting also called.
Complete the following statement. Notably the added heat to the system will involve in an increase of potential energy of the system. There are two types of vaporization.
When a substance undergoes fusion it. Melting or fusion is a physical process that results in the phase transition of a substance from a solid to a liquid. For this process to take place heat is being applied to the solid substance and the particles of the solid loose then move freely thereby reducing the intermolecular.
Heat of fusion is defined as the heat or enthalpy change when a solid substance is converted into a liquid state at its melting point. Whenever matter undergoes a. This occurs when the internal energy of the solid increases typically by the application of heat or pressure which increases the substances temperature to the melting point.
What substance dissolves most substance answer. For example Ice melts at its melting point 0 o C 273K. Fusion is a physical process.
There are two types of vaporization. When a substance boils it undergoes a process known as. Water Water is called the universal solvent because it is capable of dissolving more substances than any other liquid.
Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase. It is accompanied by the absorption of 143 kcal of heat. Melting or fusion is a physical process that results in the phase transition of a substance from a solid to a liquid.
Verb used with object dissolved dissolving. Is transferred from one body to the next.
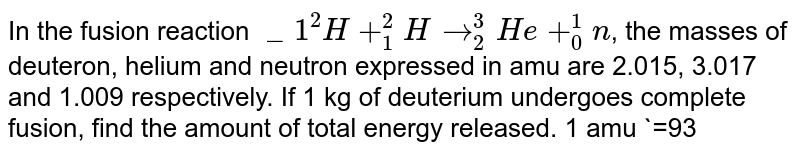
In The Fusion Reaction 1 2h 1 2hrarr2 3he 0 1n The Masses Of Deuteron Helium And Neutron Expressed In Amu Are 2 015 3 017 And 1 009 Respectively If 1 Kg Of Deuterium Undergoes Complete Fusion Find The Amount

This Work Is Licensed Under A Creative Commons Attribution 4 Ppt Download

Standard Enthalpy Changes Of Reaction Section Ppt Download
Crystals September 2021 Browse Articles

Analysis Of Cold Thermal Energy Storage Using Phase Change Materials In Freezers Sciencedirect
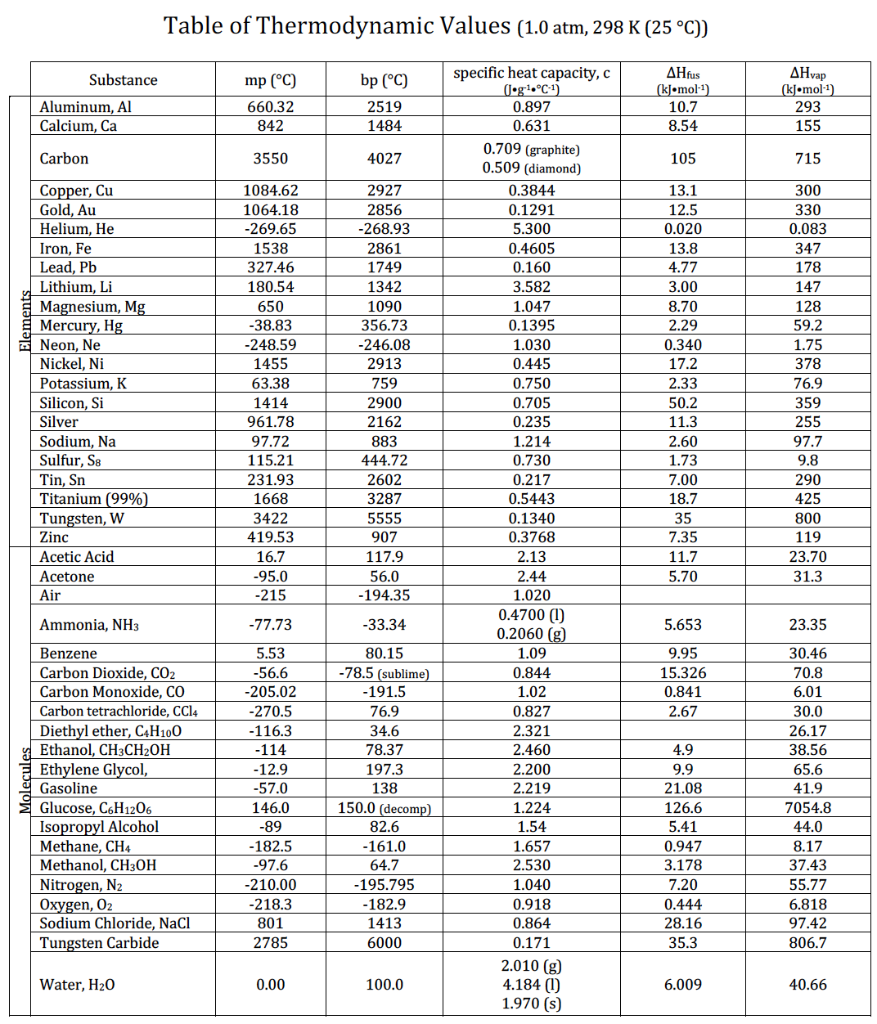
Solved 1 Using The Table Of Thermodynamics Provide An Chegg Com

What Is Latent Heat Explain With Example
Why Can Elements Heavier Than Iron Only Undergo Fission Whilst Elements Lighter Than Iron Can Only Undergo Fusion Quora
Why Can Elements Heavier Than Iron Only Undergo Fission Whilst Elements Lighter Than Iron Can Only Undergo Fusion Quora

Internal Energy And Enthalpy Springerlink
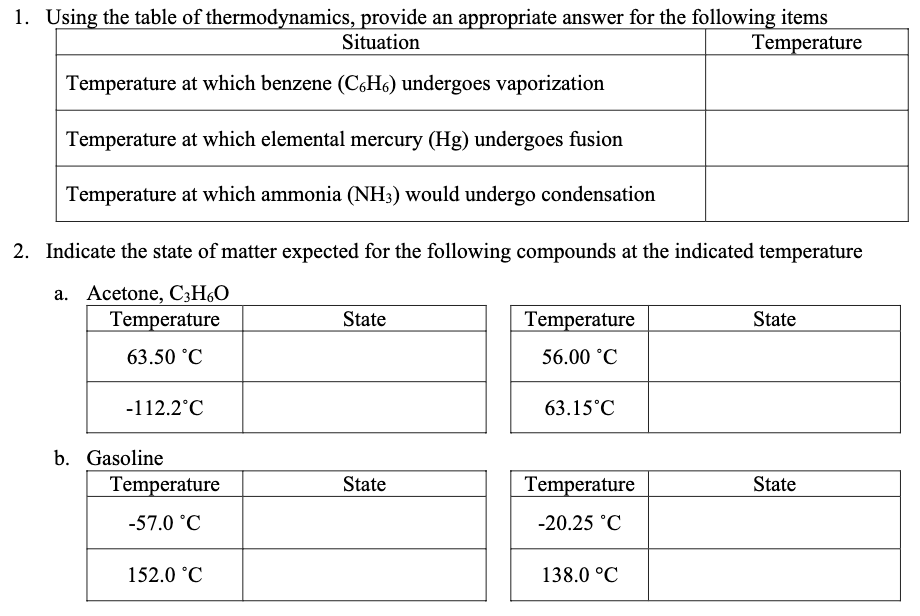
Solved 1 Using The Table Of Thermodynamics Provide An Chegg Com

Materials October 2 2021 Browse Articles

Lab Heat Of Crystallization Classroom Freebies
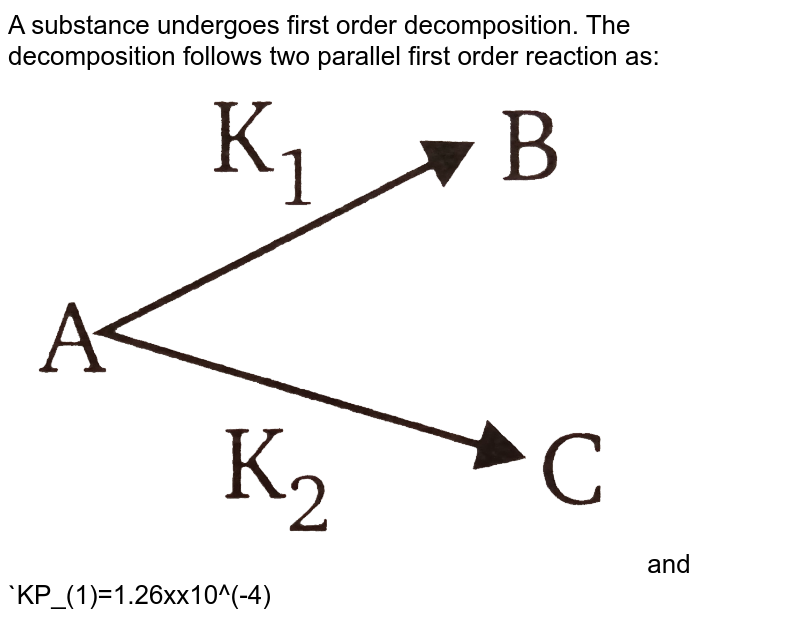
A Substance Undergoes First Order Decomposition The Decomposition Follows Two Parallel First Order Reaction As And Kp 1 1 26xx10 4 Sec 1 K 2 3 80xx10 5 Sec 1 The Percentage Distribution Of B And C Are
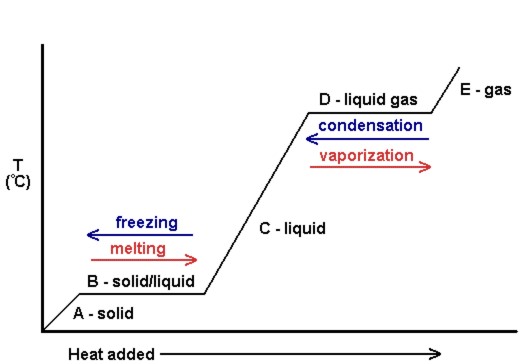
Phase Transitions Melting Boiling And Subliming Introductory Chemistry 1st Canadian Edition

Temperature And Thermal Equilibrium Ppt Download

Inner Membrane An Overview Sciencedirect Topics


